MOA
SOTYKTU has a unique MOA that selectively targets TYK21,2

How SOTYKTU works

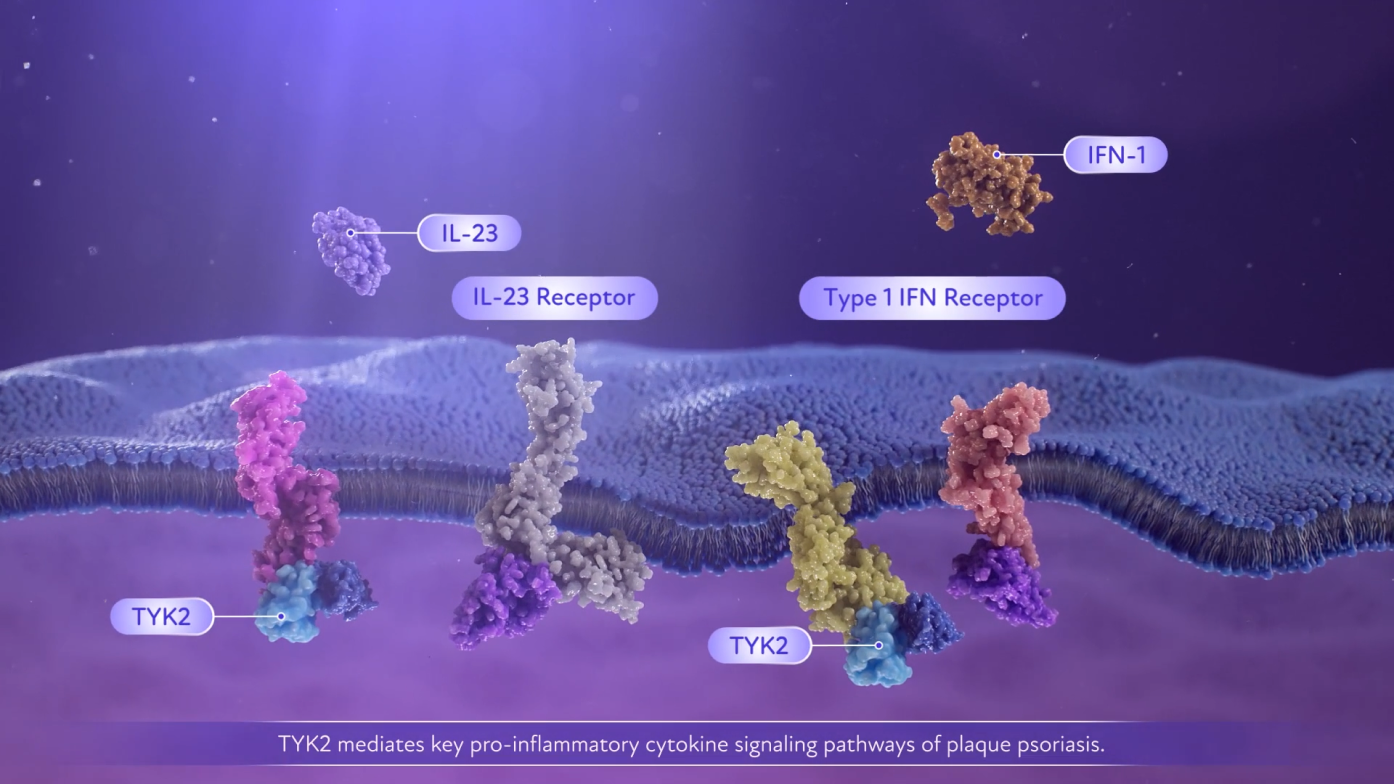
- TYK2 mediates multiple cytokine pathways, such as IL-23. By doing so, TYK2 impacts the production of IL-17. TYK2 is an important link between IL-23 and IL-17, key inflammatory cytokines in psoriasis3
- SOTYKTU is an oral, selective TYK2 inhibitor that disrupts this pathway1,2*†
- SOTYKTU is selective for TYK2 and has not been shown to inhibit JAK1/2/3 pathways at clinically relevant doses in in vitro assays1,2
The precise mechanism linking inhibition of TYK2 enzyme to therapeutic effectiveness in the treatment of adults with moderate-to-severe plaque psoriasis is not currently known.1
TYK2 pairs with JAK1 or JAK2 to mediate multiple cytokine pathways and transmit signals.1
| * | SOTYKTU reduced psoriasis-associated gene expression in psoriatic skin in a dose-dependent manner, including reductions in IL-23 pathway- and Type I IFN-regulated genes.1 |
| † | SOTYKTU reduced IL-17A, IL-19, and beta-defensin by 47% to 50%, 72%, and 81% to 84% respectively following 16 weeks of once-daily treatment.1 |
| The relationship between these pharmacodynamic markers and the mechanism(s) by which SOTYKTU exerts its clinical effects is unknown.1 | |
| Tyrosine kinase 2 is a member of the Janus kinase family.1 | |
| IFN=interferon; IL=interleukin; JAK=Janus kinase; MOA=mechanism of action; STAT=signal transducer and activator of transcription; TYK2=tyrosine kinase 2. |
SELECT IMPORTANT SAFETY INFORMATION
Potential Risks Related to JAK Inhibition: It is not known whether tyrosine kinase 2 (TYK2) inhibition may be associated with the observed or potential adverse reactions of Janus Kinase (JAK) inhibition. In a large, randomized, postmarketing safety trial of a JAK inhibitor in rheumatoid arthritis (RA), patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of all-cause mortality, including sudden cardiovascular death, major adverse cardiovascular events, overall thrombosis, deep venous thrombosis, pulmonary embolism, and malignancies (excluding non-melanoma skin cancer) were observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. SOTYKTU is not approved for use in RA.
PLEASE SEE ADDITIONAL IMPORTANT SAFETY INFORMATION BELOW.
Take a closer look at the SOTYKTU MOA and hear from your peers
Watch videos to learn about the SOTYKTU MOA and hear from Jeffrey Sobell, MD, and Scott Drew, DO, as they go deeper.
QUICK POLL

ARE YOU READY TO EXPLORE MORE? SELECT A TOPIC BELOW.
- SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11(5):1763-1776.
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009:129(6):1339-1350.


